Over the past few years, ideas about bile acids have evolved from their being seen as simple lipid solubilizers to complex metabolic integrators. Recently, synthetic agonists of the bile acid receptors have been developed to serve as next-generation drugs for the treatment of metabolic disorders and inflammatory diseases.
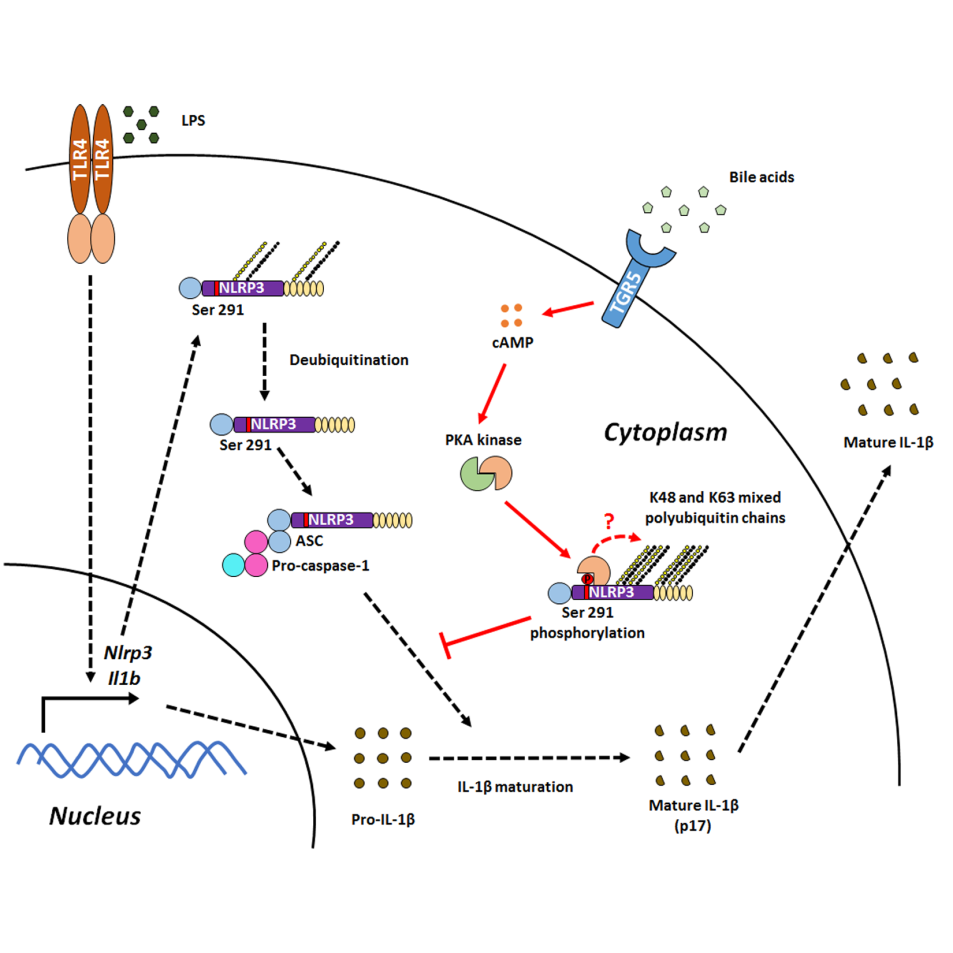
On the other hand, NLRP3 inflammasome activation is closely associated with diverse inflammatory diseases, but its regulatory networks, especially the endogenous mechanisms, remain elusive. Furthermore, how the NLRP3 inflammasome is activated and regulated in health and disease is the key issue in this field. Recent studies have shown that ubiquitination of NLRP3 serves as a critical brake on its activation, but how this ubiquitination occurs and its upstream trigger(s) are still unclear. More importantly, another critical PTM, phosphorylation, is still unreported in NLRP3.
A latest study led by Zhejiang University School of Medicine immunologists and published September 27, 2016 in Immunity, demonstrate that bile acids inhibit NLRP3 inflammasome activation via the TGR5-cAMP-PKA axis both in vitro and in vivo. Mechanistically, PKA activation induced by bile acids and TGR5 signaling phosphorylates NLRP3 on a single, evolutionarily conserved residue, Ser 291, and this leads to the ubiquitination of NLRP3. Importantly, several gain-of-function mutations in Nlrp3 related to cryopyrin-associated periodic fever syndromes (CAPS) resist PKA-induced phosphorylation and ubiquitination, potentially providing an explanation for the pathogenesis of CAPS in some patients.
Their findings suggest an unexpected endogenous inhibitory mechanism for NLRP3 inflammasome activation by bile acids via combinatorially modifying the NLRP3 protein by PKA-induced phosphorylation and ubiquitination. In light of our findings, targeting TGR5 may lead to new insights and treatments for NLRP3-related diseases.
Ph.D. students Chuansheng Guo, Shujun Xie, and Zhexu Chi share the co-first authorship, and the whole study is led by Prof. Di Wang from the Institute of Immunology at Zhejiang University School of Medicine, collaborated with Profs. Linrong Lu, Hu Hu, Zongping Xia, Yuehai Ke, and Dajing Xia. This work is supported by the National Natural Science Foundation of China.