On February 12, 2019, Professor Cai Zhijian/Professor Wang Jianli from the Institute of Immunology, Zhejiang University, published the latest research results in 《Immunity》online,which named "Specific Decrease in B Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8+ T Cell Responses”. This work fully reveals the new mechanism of systemic immunosuppression after chemotherapy, and provides a new idea for clinically improving the chemotherapeutic anti-tumor effect.
Chemotherapy is one of the most effective methods of treating cancer at present, and it is also called the three major treatments for cancer together with surgery and radiotherapy. However, chemotherapy has long been associated with the induction of systemic immunosuppression. Many chemotherapeutic drugs are myelosuppressive agents that inhibit the production of blood cells in the bone marrow, reduce absolute white blood cell counts, and cause lymphopenia. Tumor-specific CD8+ T cells are the most important effector cells for scavenging chemotherapy-resistant or residual tumor cells after chemotherapy, and can effectively prevent tumor recurrence after chemotherapy. However, the systemic immunosuppression associated with chemotherapy greatly limits the activation of tumor-specific CD8+ T cells, ultimately leading to failure of chemotherapy, tumor recurrence and rapid progression. Therefore, fully elucidating the mechanism of systemic immunosuppression after chemotherapy is of great significance for improving the effect of chemotherapy and preventing tumor recurrence.
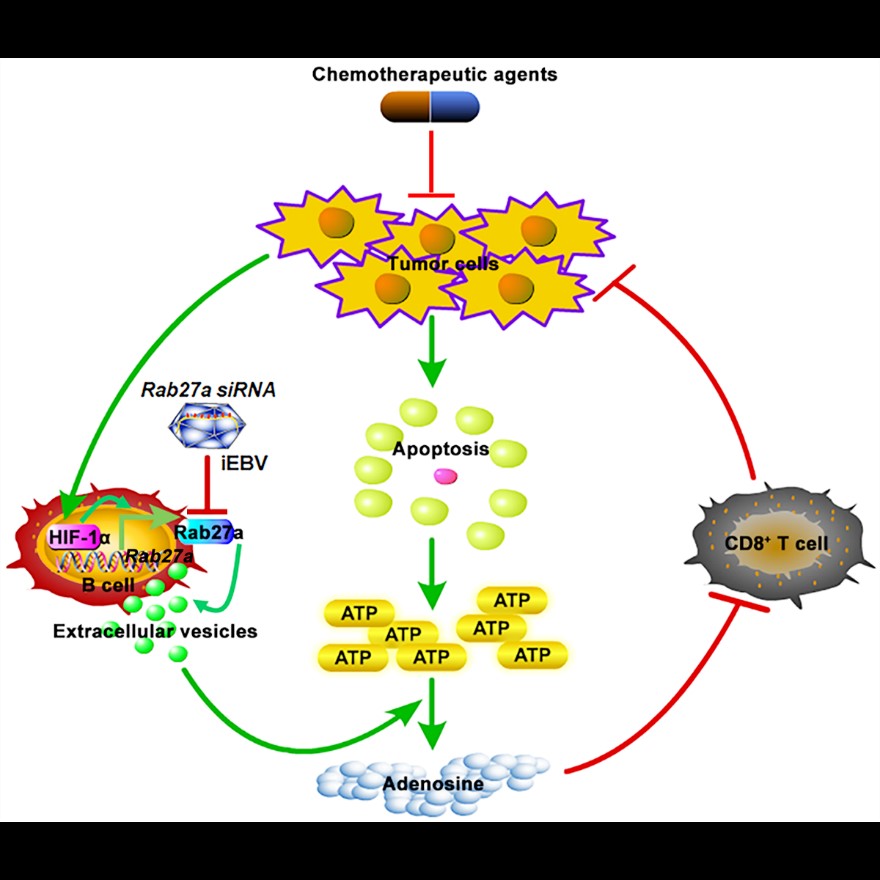
Chemotherapy-induced apoptotic tumor cells can release large amounts of ATP extracellularly in a short period of time. It is still unclear how the human body handles such a large level of ATP and its anti-tumor immunity. CD39 is an ectonucleoside triphosphate diphosphohydrolase 1-Entpd1 that hydrolyzes ATP to ADP or AMP. CD73 is an ecto-5’-nucleotidase-Nt5e that hydrolyzes AMP to adenosine. Adenosine is a potent immunosuppressive molecule that inhibits the activation of CD8+ T cells. The study found that CD19+ extracellular vesicles released by B cells contain high CD39 and CD73 molecules, which can hydrolyze ATP released by chemotherapeutic drugs to adenosine, inhibiting the activation of CD8+ T cells after chemotherapy, thereby impairing the anti-tumor effect of chemotherapy. Although CD19+ extracellular vesicles in tumor mice and normal mice contain similar CD39 and CD73 molecules and show similar ability to hydrolyze ATP and inhibit anti-tumor effects of chemotherapy, B cells from tumor mice can release more extracellular vesicles compared with normal mice. Further studies have shown that the tumor microenvironment can up-regulate the protein level of HIF-1ɑ in B cells, and then HIF-1ɑ directly binds to the promoter of Rab27a gene, promotes the expression of Rab27a protein, and finally promotes the secretion of extracellular vesicles by B cells. Similarly, compared with healthy subjects, the study showed that there were more CD19+ extracellular vesicles in serum from tumor patients with different stages, and patients with less CD19+ extracellular vesicles showed better chemotherapy effects. These work revealed significance of serum CD19-positive extracellular vesicles in the diagnosis of cancer and the evaluation of chemotherapy effects.
In addition, in order to further reveal the effect of CD19+ positive extracellular vesicles on chemotherapy, the team constructed a kind of mouse whose B cells are specifically deficient in Rab27a and some tumor models. Then a high degree of CD8+ T cell activation in the mouse was discovered after chemotherapy, and even half of the mice's tumors can completely disappear. To link this finding to clinical applications, the team used Epstein-Barr virus (EBVs) to specifically infect human B cells, introducing Rab27a small interfering RNA into inactivated EBVs (Rab27a siRNA/iEBVs). And then we use Rab27a siRNA/iEBVs to specifically inhibit the expression of Rab27a in human B cells of humanized NSG mice. We concluded that Rab27a siRNA/iEBVs significantly enhanced the efficacy of chemotherapy in tumor-bearing humanized mice and enhanced the number of human CD8 positive T cells in humanized NSG mice.
Collectively, the study identified a novel mechanism of immunosuppression after chemotherapy mediated by CD19+ extracellular vesicles and demonstrated the prospect of clinical application of Rab27a siRNA/iEBVs in combination with chemotherapy.
The work was mainly completed by Professor Cai Zhijian and Professor Wang Jianli of the Basic Medical College of Zhejiang University. Zhang Fanghui, a doctoral student of the Basic Medical College, is the first author of the thesis. Master Li Rongrong and Dr. Yang Yunshan from the Zhejiang Cancer Hospital are the co-first authors of the paper. Professor Cai Zhijian and Professor Wang Jianli are the corresponding authors of the paper. The project was supported by the National Key Research and Development Program “Property Control of Protein Machines and Life Processes”, and supported by the National “973” Program and the National Natural Science Foundation of China.