Aggrephagy,the degradation of protein aggregates via autophagy, is an important biologicalprocess to clear intracellular toxic protein aggregates. Thus, it is animportant target for the treatment of aggregates-related diseases such asneurodegenerative diseases. How autophagosomes selectively recognize proteinaggregates has always been an important issue, and the mechanism needs to befurther elucidated. Traditional aggregation-prone proteins receptors (P62,NBR1, and TAX1BP1, etc.) mediate aggrephagy by binding ubiquitin chains onaggregates and LC3 protein on the autophagosome membrane. On the other hand,receptors mediating non-ubiquitinated substrates' aggrephagy remain unknown.More importantly, currently known aggrephagy receptors selectively facilitatethe clearance of protein condensates with liquidity, rather than pathogenicprotein aggregates with little liquidity (solid aggregates). It is urgent tofind novel autophagy receptors that specifically target solid-state aggregatesto treat human diseases.
OnApril 1, 2022, Prof. YI Cong from the Zhejiang University School of BasicMedicine / The First Affiliated Hospital of the Zhejiang University School ofMedicine, in collaboration with Prof. ZHANG Min from Tsinghua University Schoolof Pharmacy, and Prof. GE Liang from Tsinghua University School of LifeSciences jointly published an article entitled “CCT2 is an aggrephagy receptorfor clearance of solid protein aggregates”in the journal Cell. The breakthroughstudy discovered a novel aggrephagy receptor CCT2, which plays a crucial rolein the clearance of solid protein aggregates.
Inthis study, the group discovered a novel aggrephagy receptor, CCT2, thatpromotes the autophagic clearance of multiple toxic protein aggregatesassociated with neurodegenerative diseases. Like traditional aggrephagyreceptors, CCT2 is able to bind to LC3 and protein aggregates. The differenceis that CCT2 binds protein aggregates in a ubiquitin-independent manner throughits apical domain, which provides a structural basis for CCT2 to specificallyrecognize aggregates.
Importantly,it was found that CCT2 and traditional aggrephagy receptors differsignificantly in their choice of aggregate state: traditional autophagyreceptors are more inclined to degrade fluid, liquid aggregates, while CCT2 ismore inclined to choose solid state aggregates. Therefore, CCT2 is more likelyto function in pathological conditions and be a drug target than knownaggrephagy receptors (Fig. 1).
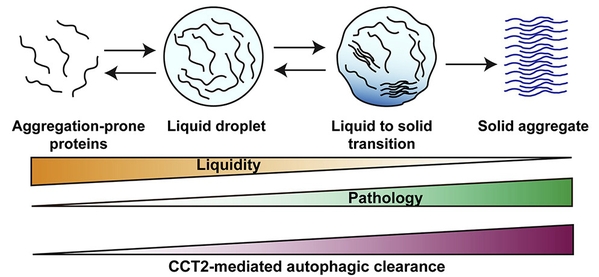
Fig.1Phase transition and consolidation of aggregates and CCT2 mediated autophagicclearance of solid aggregates
CCT2is a subunit of the Chaperonin complex, which acts as a molecular chaperone tohelp correct misfolded proteins. Further investigation revealed that CCT2mediates aggrephagy in its monomeric form, as only the monomeric form of CCT2is able to expose the VLIR domain, the binding site to LC3.
Interestingly,the presence of aggregates hindered the formation of the Chaperonin complex,thereby releasing more CCT2 monomers to facilitate aggregate clearance. Thetransition of CCT2 from a complex to a monomer shifts its function from amolecular chaperone to an autophagy receptor. This both prevents aggregation ofmisfolded proteins before aggregate formation and mediates the clearance ofaggregates after aggregation, thus providing an efficient way to maintainintracellular protein homeostasis (Figure 2).
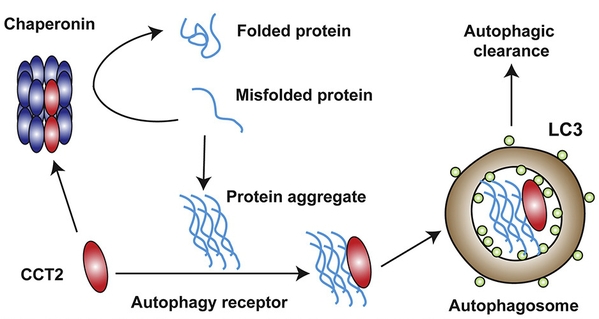
Fig.2 Functional switch of CCT2 between chaperone and autophagic receptor
Further,the structure and function of CCT2 as a solid aggregates receptor were verifiedin yeast. Both in vitro and in vivo assays showed CCT2 could bind to Atg8 (thehomolog of LC3 in yeast cells). Mutation of the conserved binding sequencebetween CCT2 and Atg8 significantly impaired the interaction of CCT2 and Atg8.Also, CCT2 is involved in the clearance of solid aggregates Ape1 P21L.
Overall,this study revealed that CCT2, as a receptor for autophagy of proteinaggregates, is highly conserved during species evolution, and prefers tomediate solid aggregates degradation. How to prevent pathological proteinaggregation has always been a challenging yet significant issue in the field ofneurodegenerative diseases. Therefore, the study has huge potential forclinical applications. Just as Prof. Yi said, “Since solid aggregates are wellrecognized as one of the pathological mechanisms of certain diseases likeAlzheimer’s disease and polyQ diseases, the discovery of CCT2 perhaps can serveas an innovative target for treating such diseases with high disease burden.”












