Oxidative phosphorylation is the majorcellular process utilized by most free-living eukaryotes to gain energy, in theform of ATP, from catabolism. It happens on the inner mitochondria membrane(IMM), where four membrane-bound complexes (complex I-IV, CI-CIV) firsttransport electron from the reductive metabolite NADH to molecular oxygen O2.Current structural studies of the above electron transport chain (ETC)complexes are limited to yeast, mammals, algae and vascular plant, belonging tothe Opisthokonta and Archaeplastida clades of eukaryotes. For other eukaryoticclades such as the Alveolates, although tomographic studies have already revealeddiversities in their mitochondrial cristae morphologies, structuralinvestigations into the ETC complexes are essentially lacking.
On March 31st 2022, Dr. ZHOU Long from theZhejiang University School of Medicine and Dr.James Letts from the Molecularand Cellular Biology Department at University of California Davis collaboratedto publish a research article in the journal Science. The study reported cryoEMstructures of a 2.3 MDa super-complex I+III2 (SC I+III2) and a 2.7 MDa complexIV dimer (CIV2) of Tetrahymena thermophila (Tt), a key ciliate model organismbelonging to the Alveolata clade, at 2.6 Å and 3.0 Å resolution respectively.Compared to the 1.4 MDa mammalian SC I+III2 with 67 subunits, Tt-SC I+III2encompasses 91 subunits, out of which 20 subunits have no reported homologues.Moreover, the Tt-CIV2 has 52 subunits per protomer and has surpassed CI as thelargest individual ETC complexes, while existing eukaryotic CIV structuresusually consist of 10-14 subunits with ~200 KDa mass.
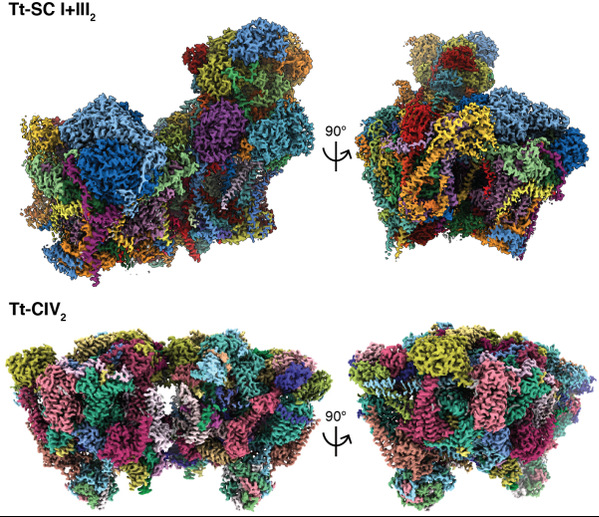
Instead of the ‘14 core subunit’architecture shared by most species’ CI, Tt-CI has three core subunits ND1, ND2and ND5 split into two poly-peptide chains. Neither the usual ‘active-deactive’activity transition nor the ‘open-close’ conformational change exists in Tt-CI,while local conformations of the loop regions near ubiquinone/co-enzyme Q (CoQ)tunnel resemble mammalian CI in the closed state. These detailed structuralinformation of the constitutively active CI suggests that ‘open-close’ statetransition as well as local CoQ loop rearrangements may not be required by theconserved mechanism of CI substrate turnover and redox-H+ pumping coupling. Tt-CI actually merges with Tt-CIII2 into anobligatory SC I+III2, the tight association pulls the two complexes closer inthe inter-membrane space (IMS), bending the joint membrane domain to fit thecurvature of Tetrahymena’s cristae IMM. This likely leads to the high degree ofsymmetry-breaking between the two CIII protomers, as the inter-heme bL distancehas increased from the highly conserved 11 Å to 14 Å, beyond the directelectron transfer distance. The physiological role of SC I+III2 formation isthus indicated to be the specialization of the two CIII2 CoQ cavities andturnover rate coupling between CI and CIII2. 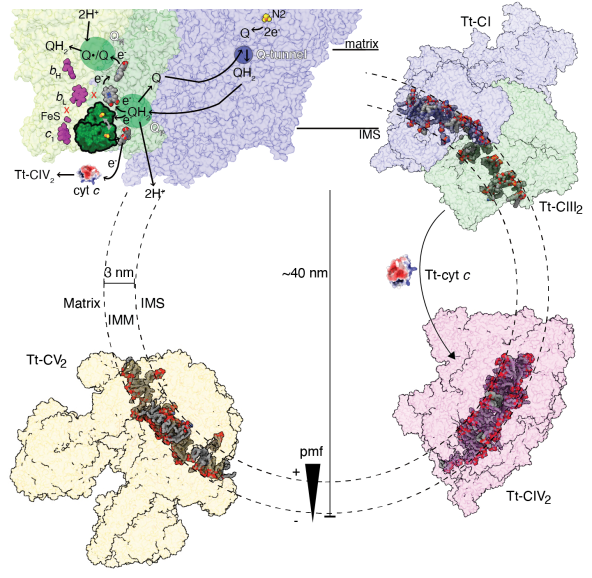
Tt-CIV2 is highly divergent as compared tothe classic 14 subunit mammalian model. Its core subunit extensions and uniqueaccessory subunits form a cytochrome c binding crate with positive surfaceelectrostatic potential, while homology modeling shows that CIV binding site ofTt cytochrome c is negatively charged. Such swapped charge as compared tomammalian CIV-cytochrome c interactions explains why Tt-CIV can’t oxidasemammalian cytochrome c. In addition, Tt-CIV2 also harbors three mitochondrialcarriers belonging to the solute carrier family 25A (SLC25A), as well as ahexameric α-propeller domain similar to the transporting chaperone formed bytranslocase of the inner membrane (TIM) 8, 9, 10 or 12. All these non-electrontransporting accessory subunits suggest that Tt-CIV2 is more than a ETCcomplex, but has evolved into a mitochondrial redox homeostasis andcross-membrane translocation hub in ciliates. “People usually think ofeukaryotes as a taxonomic combination of yeast, C. elegans, drosophila,zebrafish and mice, but there are much more populations than those. This workhas certainly demonstrated a higher level of diversity in eukaryotic ETCstructures than we used to believe, thus opened up many new possibilities inthe field of bioenergetics. Investigation into protist ETC structures and theapproach of solving multiple complex structures from one cryoEM dataset alsoprovided new angles into the pharmaceutical developments againstlife-threatening parasites including plasmodium and leishmania.”Dr. Zhou said.












