Aggrephagy, the degradation of protein aggregates via autophagy, is an important biological process to clear intracellular toxic protein aggregates. Thus, it is an important target for the treatment of aggregates-related diseases such as neurodegenerative diseases. How autophagosomes selectively recognize protein aggregates has always been an important issue, and the mechanism needs to be further elucidated. Traditional aggregation-prone proteins receptors (P62, NBR1, and TAX1BP1, etc.) mediate aggrephagy by binding ubiquitin chains on aggregates and LC3 protein on the autophagosome membrane. On the other hand, receptors mediating non-ubiquitinated substrates' aggrephagy remain unknown. More importantly, currently known aggrephagy receptors selectively facilitate the clearance of protein condensates with liquidity, rather than pathogenic protein aggregates with little liquidity (solid aggregates). It is urgent to find novel autophagy receptors that specifically target solid-state aggregates to treat human diseases.
On April 1, 2022, Prof. YI Cong from the Zhejiang University School of Basic Medicine / The First Affiliated Hospital of the Zhejiang University School of Medicine, in collaboration with Prof. ZHANG Min from Tsinghua University School of Pharmacy, and Prof. GE Liang from Tsinghua University School of Life Sciences jointly published an article entitled “CCT2 is an aggrephagy receptor for clearance of solid protein aggregates”in the journal Cell. The breakthrough study discovered a novel aggrephagy receptor CCT2, which plays a crucial role in the clearance of solid protein aggregates.
In this study, the group discovered a novel aggrephagy receptor, CCT2, that promotes the autophagic clearance of multiple toxic protein aggregates associated with neurodegenerative diseases. Like traditional aggrephagy receptors, CCT2 is able to bind to LC3 and protein aggregates. The difference is that CCT2 binds protein aggregates in a ubiquitin-independent manner through its apical domain, which provides a structural basis for CCT2 to specifically recognize aggregates.
Importantly, it was found that CCT2 and traditional aggrephagy receptors differ significantly in their choice of aggregate state: traditional autophagy receptors are more inclined to degrade fluid, liquid aggregates, while CCT2 is more inclined to choose solid state aggregates. Therefore, CCT2 is more likely to function in pathological conditions and be a drug target than known aggrephagy receptors (Fig. 1).
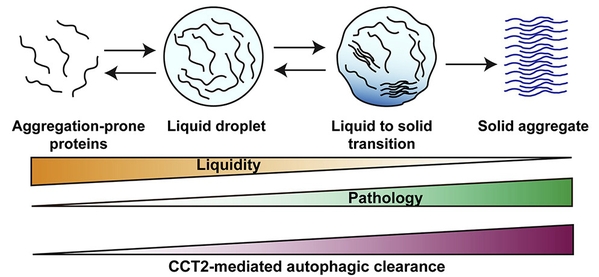
Fig.1 Phase transition and consolidation of aggregates and CCT2 mediated autophagic clearance of solid aggregates
CCT2 is a subunit of the Chaperonin complex, which acts as a molecular chaperone to help correct misfolded proteins. Further investigation revealed that CCT2 mediates aggrephagy in its monomeric form, as only the monomeric form of CCT2 is able to expose the VLIR domain, the binding site to LC3.
Interestingly, the presence of aggregates hindered the formation of the Chaperonin complex, thereby releasing more CCT2 monomers to facilitate aggregate clearance. The transition of CCT2 from a complex to a monomer shifts its function from a molecular chaperone to an autophagy receptor. This both prevents aggregation of misfolded proteins before aggregate formation and mediates the clearance of aggregates after aggregation, thus providing an efficient way to maintain intracellular protein homeostasis (Figure 2).
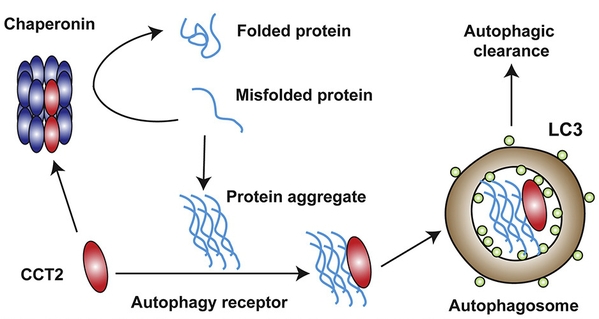
Fig. 2 Functional switch of CCT2 between chaperone and autophagic receptor
Further, the structure and function of CCT2 as a solid aggregates receptor were verified in yeast. Both in vitro and in vivo assays showed CCT2 could bind to Atg8 (the homolog of LC3 in yeast cells). Mutation of the conserved binding sequence between CCT2 and Atg8 significantly impaired the interaction of CCT2 and Atg8. Also, CCT2 is involved in the clearance of solid aggregates Ape1 P21L.
Overall, this study revealed that CCT2, as a receptor for autophagy of protein aggregates, is highly conserved during species evolution, and prefers to mediate solid aggregates degradation. How to prevent pathological protein aggregation has always been a challenging yet significant issue in the field of neurodegenerative diseases. Therefore, the study has huge potential for clinical applications. Just as Prof. Yi said, “Since solid aggregates are well recognized as one of the pathological mechanisms of certain diseases like Alzheimer’s disease and polyQ diseases, the discovery of CCT2 perhaps can serve as an innovative target for treating such diseases with high disease burden.”










