近日,柯越海课题组与邵逸夫医院团队合作在Oncogene杂志上发表"Myeloid-restricted ablation of Shp2 restrains melanoma growth by amplifying the reciprocal promotion of CXCL9 and IFN-γ production in tumor microenvironment"研究论文,揭示了酪氨酸磷酸酶Shp2通过抑制巨噬细胞分泌趋化因子CXCL9,下调T细胞介导的抗肿瘤免疫应答的效应。该研究首次阐述了靶向Shp2磷酸酶对肿瘤相关巨噬细胞与微环境调控意义。
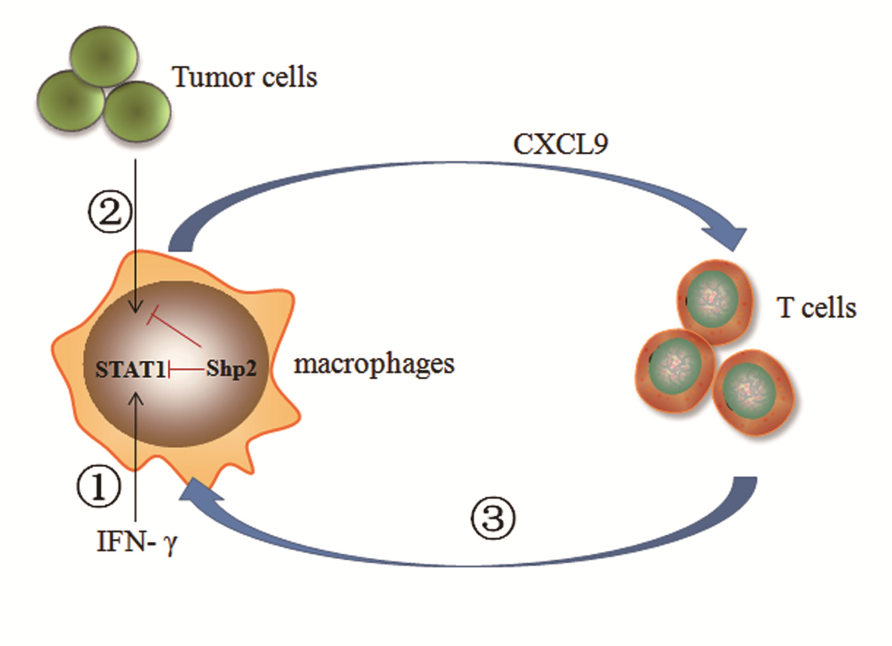
Working model. Shp2 ablation in macrophages creates a Th1-dominant tumor immune microenvironment, characterized by enhanced production of CXCL9 and IFN-γ, as well as increased infiltration of CD8+ T cells and Th1 cells. At least three mechanisms are involved in this process: Loss of Shp2 potentiates CXCL9 production in macrophages in response to ①IFN-γ and ②tumor cell secreted cytokines. ③The resultant promotion in the recruitment of IFN-γ+ T cells in turn support CXCL9 expression in TAMs.











