On Feb 14, 2018, Nature published online two Research Articles side-by-side from Professor Hailan Hu’s team in the Medical School of Zhejiang University. They reported a series of discoveries pertaining the rapid antidepressant mechanism of ketamine, the abnormal neural coding pattern and neuron-glia interaction underlying depression.
Classical antidepressants have suffered from the problems of slow action (>6~8 weeks), low efficacy (<30% emission rate) and side effects. The party drug ketamine has recently emerged as a “miracle drug” due to its rapid antidepressant actions and high efficacy on treatment-resistant depression patients. However, the mechanisms of how ketamine acts so fast,and the exact target of ketamine -- which brain region and which cell groups -- have remained elusive. Due to its addictive nature and potential side effects, ketamine has not been widely used in clinics. Therefore, elucidating the underlying mechanism of ketamine is essential for developing new rapid-acting antidepressants without side effects.
In the first article from Hailan Hu’s team, they found that ketamine blocks bursting activity in the lateral habenula (LHb), an anti-reward center in the brain, to rapidly relieve depression. LHb is a key hub that links the limbic forebrain and the midbrain monoamine centers. It is activated by negative emotions and suppresses the activity of reward-related dopaminergic and serotonergic neurons. Although it is well recognized that LHb becomes hyperactive during depression, it was a mystery how LHb encodes depression-related information. Hu's team discovered that in various animal models of depression, the intrinsic burst firing activity of LHb neurons is significantly increased. Burst firing, a particular spike pattern with clusters of high-frequency spikes, provides a robust form of information coding and a stronger output from LHb to inhibit the reward-related dopaminergic and serotonergic centers. The team discovered that LHb burst firing strongly depends on NMDAR, a major subtype of excitatory glutamate receptors within the brain. Ketamine, as a NMDAR antagonist, completely eliminates LHb bursting in vitro and reduces bursting of depressive-like animals to the level of naïve control animals in vivo. Consistently, local infusion of ketamine or another drug blocking NMDAR into LHb is sufficient to rapidly relieve depression-like symptoms including anhedonia and behavioral despair in rats. To further determine whether LHb burst firing is sufficient to mediate depression, Hu’s team applied optogenetics to express a light-sensitive channel specifically in the LHb, and showed that light-induced burst firing instantly induces depression-like behaviors in animals. Furthermore, through electrophysiology and biophysical modeling, the team found that LHb burst firing not only requires NMDAR, but also the synergistic activation of a calcium channel T-VSCC and membrane hyperpolarization. Strikingly, systemic or local blockade of T-VSCC within LHb also induces rapid antidepressant effects similar to ketamine. Collectively, these data suggest a simple and novel mechanism whereby ketamine quickly elevates mood by instantaneously blocking NMDAR-dependent bursting activity of LHb to disinhibit downstream dopaminergic and serotonergic neurons.
In the accompanying research article, Hailan Hu's team further identified the molecular mechanism by which neuron-glia interaction at the LHb regulates burst firing in depression. Ten years ago, in a collaboration with John Yates’ lab from the Scripps Institute, Hu’s team set out to search for molecules that change expression level in the LHb of congenitally depressed rats (made by Fritz Henn’s lab). Through an unbiased, mass spectrometry-based, quantitative proteomic screening, the team identified several molecules upregulated in depression and published about one of them, the bCaMKII kinase, in 2013 (Li et al., Science). In this Nature article, the team continued to characterize another upregulated candiate, the inward-rectifying potassium channel Kir4.1, which is exclusively expressed in the astrocytes, one type of glia cells, in the LHb. Through immuno-histochemistry and electron microscopy, they uncovered a striking expression pattern of Kir4.1 in the LHb: unlike in hippocampus, Kir4.1-positive astrocytic processes closely envelope the soma of a majority of LHb neurons. This led to the hypothesis that Kir4.1 may efficiently regulate neuronal firing by buffering the extracellular potassium within this narrow extracellular space. Indeed, electrophysiological recording and biophysical modeling verified that the level of Kir4.1 on astrocytes tightly regulates the degree of membrane hyperpolarization and amount of burst activity of LHb neurons. Specifically, overexpression of Kir4.1 in LHb astrocytes led to neuronal hyperpolarization and increased bursting. Vice versa, acute blockade or viral-mediated knockdown of Kir4.1 in astrocytes caused neural depolarization and eliminated intrinsic burst firing of LHb neurons. Correspondingly, gain- or loss- of Kir4.1 function in the LHb astrocytes drove or suppressed depressive behaviors, respectively. These results point to the important role of a glia-specific ion channel in the regulation of neuronal firing pattern, and provide compelling evidence for Kir4.1 being a powerful molecular determinant of depression.
The two articles, with one addressing how LHb neurons increase burst firing in depression and the other revealing this burst firing to be a prominent target of rapid antidepressant ketamine, make a strong case that the firing mode of LHb neurons is critical in depression. The newly discovered structure-function mechanism at the glial-neural interface in burst regulation may enrich our understanding of the basic cell biology of how glia regulates neural activity in physiological and pathological states. Together, they provide a new framework for understanding the molecular, cellular and circuit mechanism of depression and shed important light on developing new rapid-acting antidepressants.
Both projects were carried out by post-docs Yihui Cui, Yan Yang and Kangning Sang, graduate students Yiyan Dong, Zheyi Ni and Shuangshuang Ma, under the supervision of Prof. Hailan Hu. Shengxi Wu’s team from the Fourth Military Medical University performed the immuno-electron microscope experiments. Hugues Berry’s team from CNRS in France participated in constructing the biophysical model for potassium buffering. Other collaborators include Yuezhou Li and Ying Shen‘s teams from Zhejiang University. Lushan Yu’s lab performed LC-MS measurement of ketamine. The authors wish to thank Shumin Duan for his inspiring leadership and generous support. The project was supported by grants from the National Science Foundation, the National Key R&D Program of China, the Strategic Priority Research Program (B) of the Chinese Academy of Sciences, and 111 project.
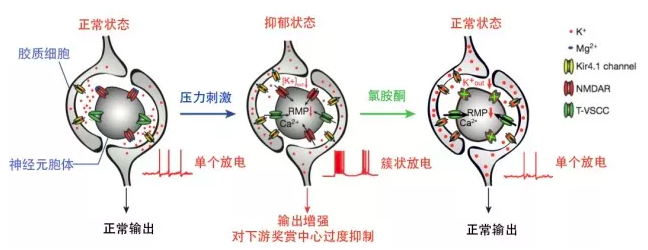
A model for mechanisms of depression and ketamine treatment in LHb: Upregulation of Kir4.1 on astrocytic processes surrounding neuronal soma leads to enhanced K+ buffering at the tight neuron-glia junction, decreased Kout and hyperpolarized neuronal RMP. Consequently, de-inactivation of T-VSCCs initiates NMDAR-dependent bursts, causing a stronger output of LHb to trigger depression. Ketamine blockade of NMDARs stops bursts and relieves depression.

Authors of the papers. From left to right are Kangning Sang, Yan Yang, Hailan Hu, Yiyan Dong, Yihui Cui, Zheyi Ni.