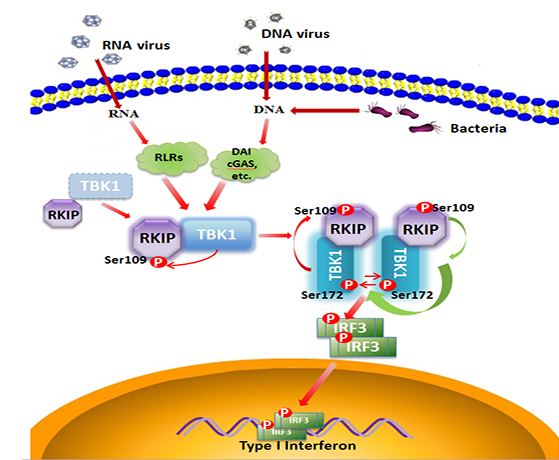
Across all signalling pathways involved in type I interferon production in innate immunity, TBK1 plays a central role in diverse processes involving Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NODs), C-type lectins and double-strand DNA (dsDNA) receptors. The activation of TBK1 in response to virus infection lies at the heart of these physiological processes. However, how TBK1 activation is finely regulated remains a topic of active debate.
Raf kinase inhibitory protein (RKIP) is the prototype member of the PEBP family and functions in a variety of physiological processes, including cell differentiation, migration, apoptosis and cell cycle, via regulating MAPK, G protein coupled receptor (GPCR), NF-κB and GSK3β signalling transduction.
In a recent paper published in EMBO Journal, Dr. Xiaojian Wang’s laboratory found that the TBK1-RKIP develops a positive-feedback for triggering TBK1 fully activation during antiviral innate immunity. RKIP is specifically phosphorylated at S109 by TBK1 upon viral infection,and the phosphorylated RKIP in turn interacts with TBK1 and promotes TBK1 activation, the fully activated TBK1 ultimately leads to the production of type I interferon to clear the invading virus . RKIP deficient mice produce lower levels of IFN-β in serum than wild type littermates in response to infection of RNA virus VSV or DNA virus HSV1. VSV titer or infiltration of inflammatory cells in liver and lung from RKIP deficient mice is significantly enhanced compared to that in organs from wild type mice. Consistently, RKIP deficient mice are more susceptible to lethal VSV and HSV infection compared to wild type mice in overall survival assays.
In summary, we have shown that RKIP is specifically phosphorylated at S109 by TBK1 upon viral infection and RKIP regulates TBK1 activation through a positive feedback mechanism. These findings not only provide insight into the positive regulation of TBK1-mediated antiviral innate immune responses, but also provide a novel post translational modification mechanism that allows RKIP to act as an anti-virus agent.
This work was supported by grants from the National Basic Research Program of China (973) (2014CB542101), the National Natural Science Foundation of China (31570864, 81230014), and the Natural Science Foundation of Zhejiang Province (LR13C080001).
http://emboj.embopress.org/cgi/doi/10.15252/embj.201694060